Can TMS Help with OCD? Treatment Options in New Jersey

Quick takeaway
Yes - TMS can be an effective option for some people with obsessive–compulsive disorder (OCD). In particular, deep TMS (dTMS) has received FDA clearance for OCD and is provided using specific, evidence-based protocols. At Elevium, TMS for OCD is offered as part of an integrated care plan that pairs neuromodulation with therapy, medication management, and careful clinical oversight.
How TMS treats OCD - the basic idea
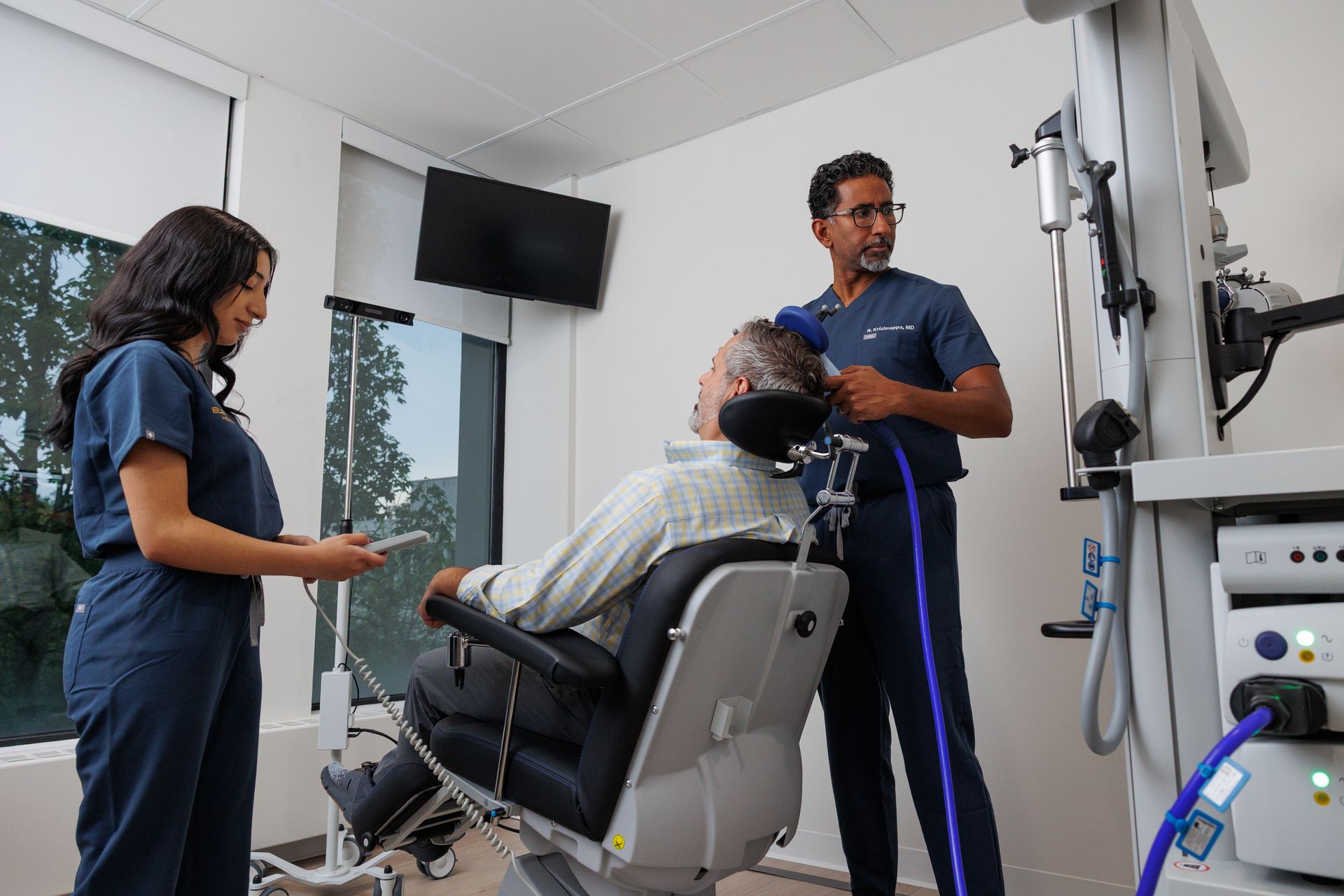
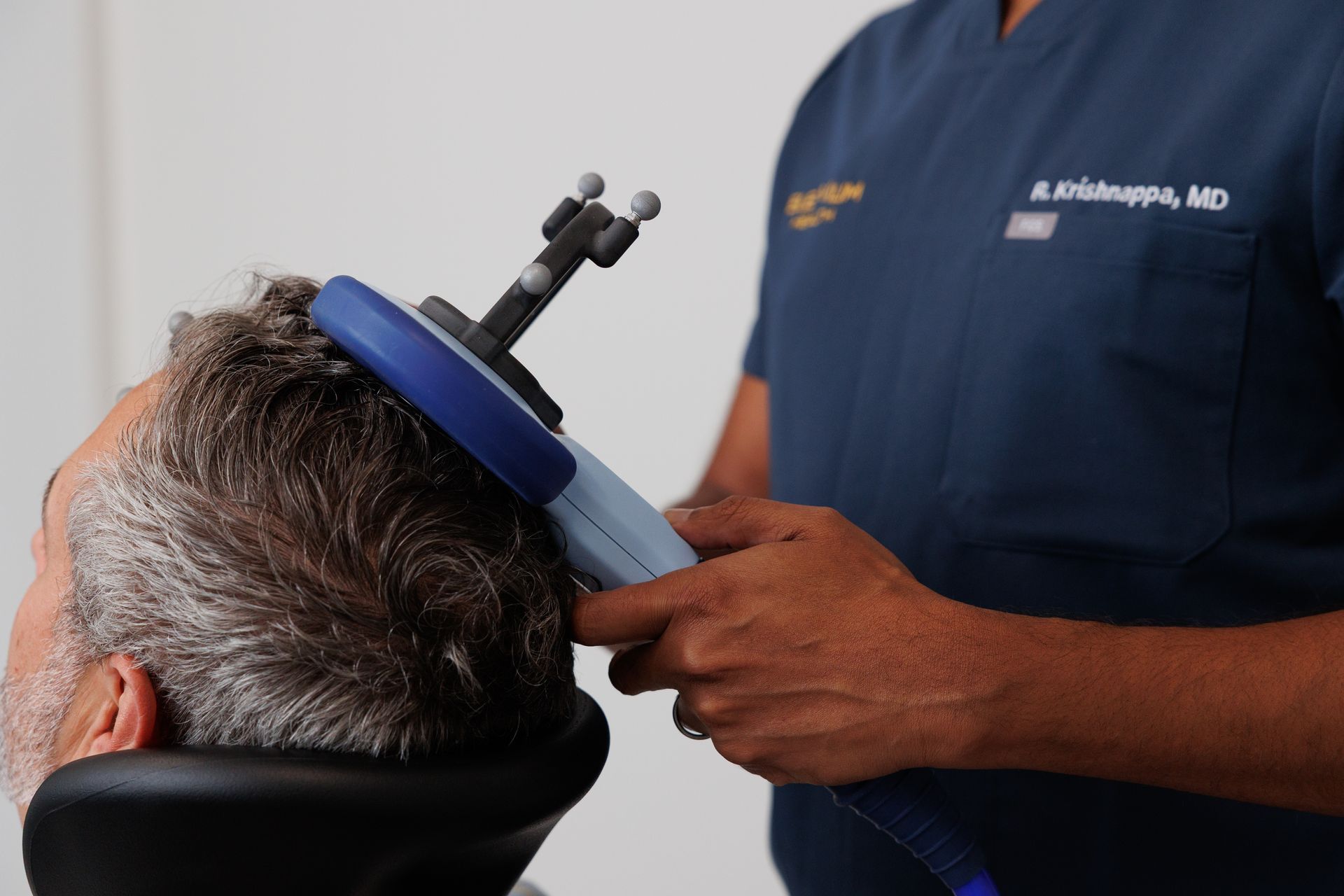
TMS uses focused magnetic fields to change activity in brain circuits involved in mood and compulsive thinking. For OCD, clinicians target the networks thought to underlie obsessive thoughts and repetitive behaviors. Deep TMS (dTMS) uses a specialized coil to reach deeper brain structures implicated in OCD and is the modality that has been FDA-cleared for this indication. Clinical protocols are standardized so clinicians can safely and reliably deliver therapeutic stimulation.
FDA approvals & what they mean
The FDA has cleared specific dTMS systems for the treatment of OCD, which means there is a regulatory basis and controlled clinical evidence supporting its safety and effectiveness for eligible patients. FDA clearance also helps establish standardized treatment protocols and safety monitoring that clinics follow.
Typical protocols & what a course looks like
- Intake & screening: A full psychiatric and medical evaluation is performed to confirm the OCD diagnosis, review prior treatments (therapies and medications), and screen for contraindications (for example, certain implants or seizure risk).
- Treatment schedule: OCD TMS protocols are typically delivered as a course of daily sessions over multiple weeks. The length of each session and the total number of visits depend on the specific dTMS protocol used and the patient’s clinical response.
- Monitoring & follow-up: Symptoms are tracked with standardized scales; clinicians adjust the plan and recommend complementary therapies (e.g., ERP — exposure and response prevention) as needed. Elevium’s guidance emphasizes standardized protocols and close outcome tracking to optimize results.
Success rates & evidence (what research and clinic experience show)
Clinical trials and real-world clinic reports show that many patients with OCD experience meaningful symptom reductions after a full course of dTMS, and some achieve remission. Outcomes vary by individual factors (severity, duration of illness, prior treatment response). Elevium’s TMS for OCD materials summarize published evidence and emphasize honest, individualized expectations during the consent process.
Patient experience at Elevium — what to expect
At Elevium the TMS experience for OCD is built around patient comfort, safety, and integration with other care:
- Personalized evaluation: Licensed psychiatrists and clinical staff perform a careful assessment to confirm candidacy and create an individualized plan.
- Experienced clinical team: Elevium’s site and staff bios show dedicated TMS technicians and clinicians who specialize in neuromodulation and patient-centered care — from intake through follow-up. Patients meet trained technicians and receive detailed education about what to expect during sessions.
- Integrated care model: TMS is offered alongside evidence-based psychotherapy (including ERP), medication management, and care coordination. Elevium emphasizes combining neuromodulation with behavioral therapy to maximize gains.
- Practical logistics: Elevium helps with benefits checks, prior authorization assistance, appointment scheduling, and outcome tracking so patients and families understand costs, time commitments, and likely next steps. (See Elevium’s service planning for New Jersey.)
Who is a good candidate?
TMS for OCD is generally considered when:
- The patient has a confirmed OCD diagnosis and persistent symptoms despite recommended first-line treatments, especially adequate trials of ERP (exposure and response prevention) and medication (usually SSRIs).
- There are no medical contraindications (screened during intake).
- The patient and family accept the time commitment of frequent clinic visits and the plan to pair neuromodulation with ongoing therapy when indicated. Elevium’s clinicians walk families through candidacy criteria in detail during the evaluation.
Risks & side effects
- Most common: Scalp discomfort or headache during or after sessions.
- Rare but important: Seizure risk is very low when patients are properly screened and protocols are followed.
- Other: Some patients report temporary fatigue or transient changes in sensation. Elevium follows device-specific safety checks and provides pre-treatment counseling so risks are understood and monitored.

Insurance & coverage in New Jersey
Coverage for TMS depends on the insurer and the specific indication. Because dTMS for OCD has FDA clearance, many insurers may consider coverage when clinical criteria are met, but policies vary and prior authorization is commonly required. Elevium’s team assists with benefits checks and prior authorizations to clarify likely out-of-pocket costs. The Elevium sitemap and practice materials reflect a focus on New Jersey services and local coverage navigation.
How to get started at Elevium (New Jersey)
- Request an evaluation - Elevium schedules a comprehensive psychiatric intake to confirm diagnosis and review prior therapy/medication history.
- Clinical screening - medical history, seizure risk assessment, and review of any implants or devices.
- Shared decision-making - the team reviews the dTMS protocol, expected timeline, benefits vs risks, and integration with ERP/psychotherapy.
- Authorization & scheduling — Elevium assists with insurance checks and plans the treatment course and follow-up.
- Treatment & measurement — daily sessions as prescribed with outcome tracking and therapy coordination.
Is TMS a first-line treatment for OCD?
No. First-line care for OCD typically includes ERP therapy and medications (SSRIs). dTMS is a valuable option when standard treatments are insufficient or not tolerated.
How long before I might feel better?
Some patients notice changes early, but most assessments are made after a full treatment course. Elevium uses standardized symptom tracking to monitor progress and adjust plans.
Can I combine TMS with ERP or medications?
Yes - combining dTMS with ERP and medication management is common and often recommended to improve outcomes. Elevium’s integrated approach emphasizes this coordination.
Share Article