TMS Therapy Near Me: Your Complete Guide to TMS in New Jersey
What is TMS (Transcranial Magnetic Stimulation)?
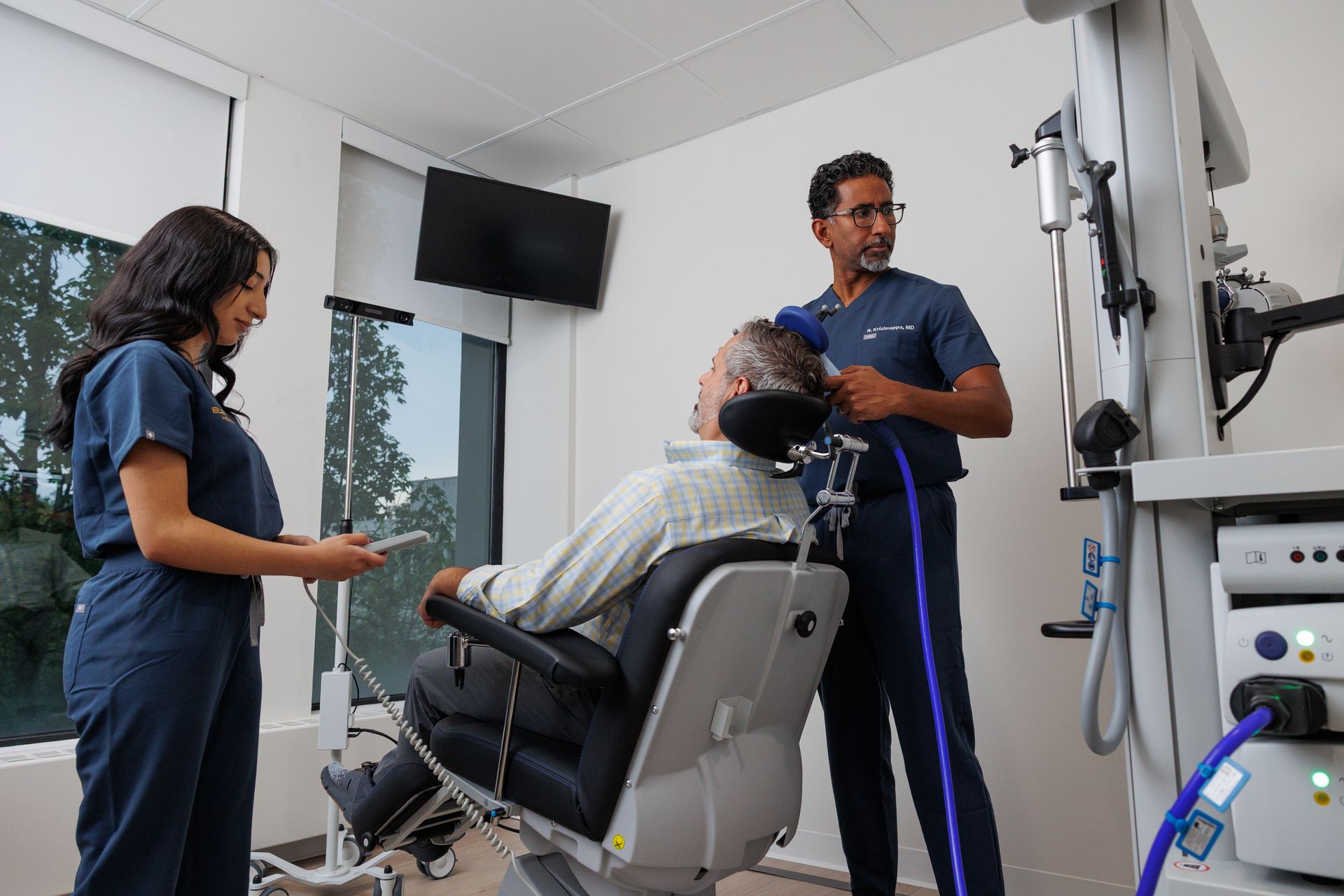
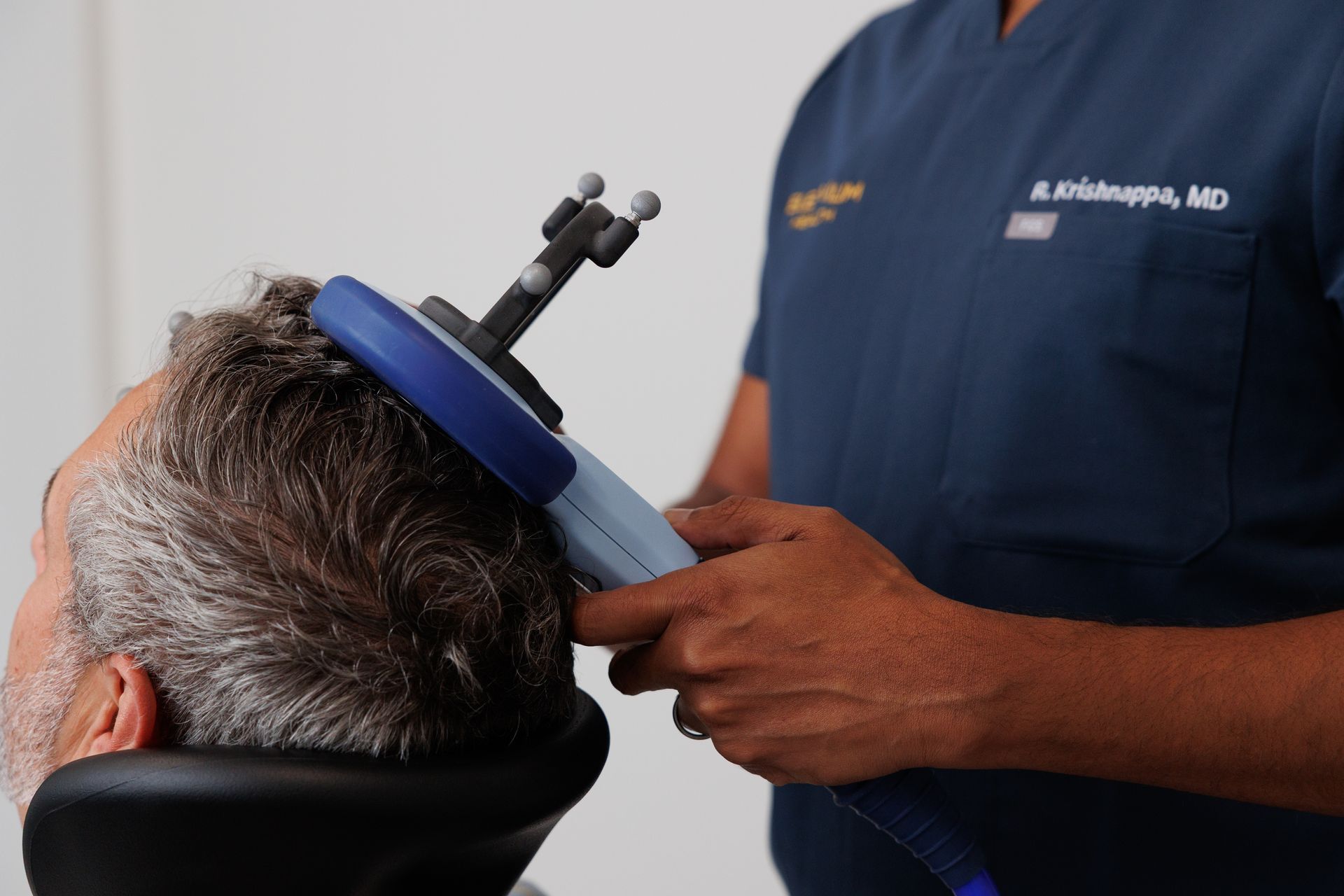
TMS uses focused magnetic pulses to stimulate targeted areas of the brain (most commonly the left dorsolateral prefrontal cortex for depression). Pulses are delivered with a coil placed on the scalp; treatment is done while awake and does not require anesthesia.
- Variants:
- Standard rTMS: sessions ~30–45 minutes.
- iTBS (intermittent theta burst stimulation): accelerated protocol with much shorter sessions (often 3–10 minutes) that can produce similar clinical results in many patients.
Safety & Side Effects:
The most common side effects are mild scalp discomfort or headache, which typically resolve. Serious events (seizures) are rare when patients are properly screened and protocols followed.
Who is a candidate for TMS?
TMS is typically considered when:
- A patient has major depressive disorder that has not responded adequately to multiple antidepressant trials or therapy.
- Medication side effects are intolerable, or the patient prefers a non-drug option.
- The patient can attend frequent sessions and has no contraindications (e.g., certain metal implants near the head, unshielded intracranial hardware, or an active condition that raises seizure risk).
- For teens: certain devices have adolescent indications — candidacy is determined by device labeling and clinician judgment.
Typical pre-treatment evaluation includes: psychiatric assessment, medical history, medication review, seizure risk evaluation, and review for metal implants. A baseline symptom scale (PHQ-9, etc.) is often used to track progress.
What to expect during treatment (timeline & session flow)
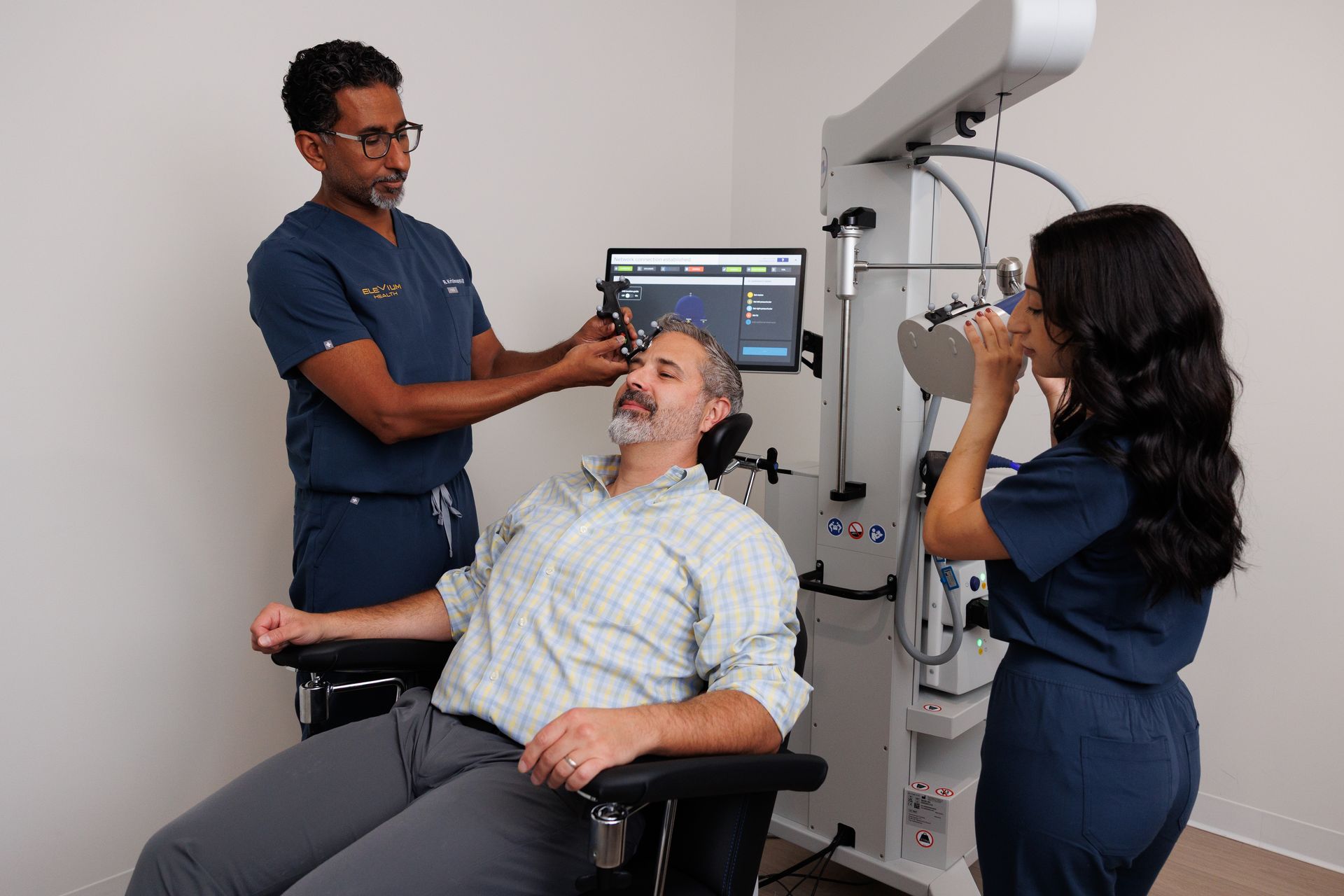
Evaluation & prep
- Initial consult / intake (60–90 minutes): psychiatric history, prior treatments, informed consent, and screening.
- Motor threshold titration: first appointments set the stimulation intensity based on each patient’s motor threshold (a quick calibration).
Treatment course
- Frequency: Most standard protocols are 5 sessions per week (Mon–Fri).
- Duration: Typical acute course is 4–6 weeks (totaling ~20–30 sessions). Some protocols extend to 6 weeks or include additional sessions for partial responders.
- Session length: Traditional rTMS sessions 20–45 minutes; iTBS sessions about 3–10 minutes.
- Maintenance: Some patients have periodic maintenance TMS (weekly or monthly) if symptoms recur.
During a session
- You’ll sit comfortably. The technician positions the coil and runs the protocol. You’re awake and alert and can read, use your phone, or relax. Most patients return to normal activities immediately after (unlike treatments that require post-procedure monitoring or sedation).
Expected results & outcomes
- When patients improve: Some patients report improvement within 2–4 weeks; others require a full course before meaningful change.
- Clinical outcomes: Studies have shown that many patients experience significant symptom reduction and improved functioning after completing a full course. Response and remission rates vary by population, past treatment history, and the protocol used. Your clinician will review expected response rates for the chosen protocol and track progress with standardized measures.
- Durability: Responses can be durable, but some patients benefit from maintenance sessions or adjunctive therapies (therapy, meds).
TMS vs Spravato vs Medication: a practical comparison
| Feature | TMS | Spravato (esketamine) | Typical oral medications (SSRIs, SNRIs, etc.) |
|---|---|---|---|
| Mechanism | Magnetic stimulation of brain circuits | NMDA-receptor modulator (esketamine) | Monoamine modulation (serotonin, norepinephrine) |
| FDA status for depression | FDA-cleared for major depressive disorder (various devices/protocols) | FDA-approved for treatment-resistant depression (REMS program) | FDA-approved agents for depression |
| Setting | Outpatient clinic; awake | In-clinic dosing + 2+ hour observation (REMS) | Outpatient, pharmacy dispensed |
| Session time | 3–45 min (daily sessions) | Single dosing session + ≥2 hours monitoring | Daily at home |
| Onset of action | Often weeks (some improvement in 2–4 weeks) | Often rapid (days to weeks) | Usually weeks (4–8 weeks to full effect) |
| Common side effects | Headache, scalp discomfort; rare seizure | Dissociation, sedation, BP rise; must monitor | GI upset, sexual side effects, sleep changes |
| Insurance landscape | Many insurers cover for treatment-resistant depression with prior auth | Often covered with prior auth if criteria met; REMS adds documentation | Usually covered; generics lower cost |
| Driving/return to activities | Usually immediate | Patients cannot drive post-dose until cleared | No restrictions except side-effects |
Which to choose?
- TMS is appealing when a non-systemic, non-sedating option is preferred, when meds haven’t worked, or when patients cannot tolerate systemic side effects.
- Spravato may be preferred for rapid relief (e.g., severe suicidal ideation in a monitored setting) or when previous trials make esketamine a good option.
- Oral meds remain first-line for many patients due to ease and low cost, but may be ineffective or poorly tolerated for some.
In many cases, these options are complementary, and clinicians may combine treatments (e.g., TMS with medication) to achieve the best outcome.
Insurance, cost & coverage in New Jersey
TMS coverage:
Many insurers cover TMS for treatment-resistant depression when prior authorization and documentation of failed medication trials are provided. Coverage policies vary by carrier. Ask your plan about specific criteria (number of failed antidepressant trials, documentation requirements)
Spravato coverage:
Frequently covered for labeled indications, but requires prior authorization and REMS-compliant administration; patient responsibility depends on plan.
Out-of-pocket options:
When coverage is denied (particularly for off-label ketamine), clinics offer self-pay pricing or financing.
What Elevium can do:
Assist with benefits checks, submit prior authorizations, provide itemized cost estimates, and advise on in-network clinic options in NJ.
Safety & contraindications
General screening includes:
- Metal implants in or near the head (some implants are disqualifying).
- History of seizure or conditions that lower seizure threshold.
- Certain medications may increase seizure risk.
- Pregnancy: TMS is non-systemic and sometimes considered, but discuss risks/benefits with your clinician.
Side effects & what to watch for:
Headaches, scalp discomfort, fatigue. Emergency signs like seizures are rare but require immediate attention.
Can I drive after TMS?
Yes — most patients return to normal activities immediately after standard TMS sessions.
How many TMS sessions will I need?
A typical acute course is 20–30 sessions over 4–6 weeks; your clinician will tailor this.
Will insurance cover TMS in New Jersey?
Many plans cover TMS for treatment-resistant depression with prior authorization; check with your insurer and Elevium for a benefits check.
Is TMS painful?
It can cause tapping sensations and occasional headaches, generally well tolerated and manageable.
Can TMS be used with antidepressant medications?
Yes - TMS is commonly used alongside medications and psychotherapy.
Share Article