Is TMS or Spravato Safe for Teens? What Parents Need to Know
Quick answer
If you’re a parent looking at TMS (transcranial magnetic stimulation)or Spravato(esketamine nasal spray) for a teen with depression, the biggest takeaway is this:
- TMS has FDA-cleared options for some adolescents (ages 15–21) as an adjunct treatment for major depressive disorder, depending on the specific TMS system and clinical scenario.
- Spravato is not established for pediatric patients (safety/effectiveness in people under 18 hasn’t been established in labeling).
- Spravato also requires in-clinic administration and at least 2 hours of monitoring each session due to risks like sedation/dissociation and respiratory depression.
Below is a parent-friendly guide: eligibility, safety data, FAQs, and what options a clinic like Elevium may offer for younger patients.
What are these treatments?
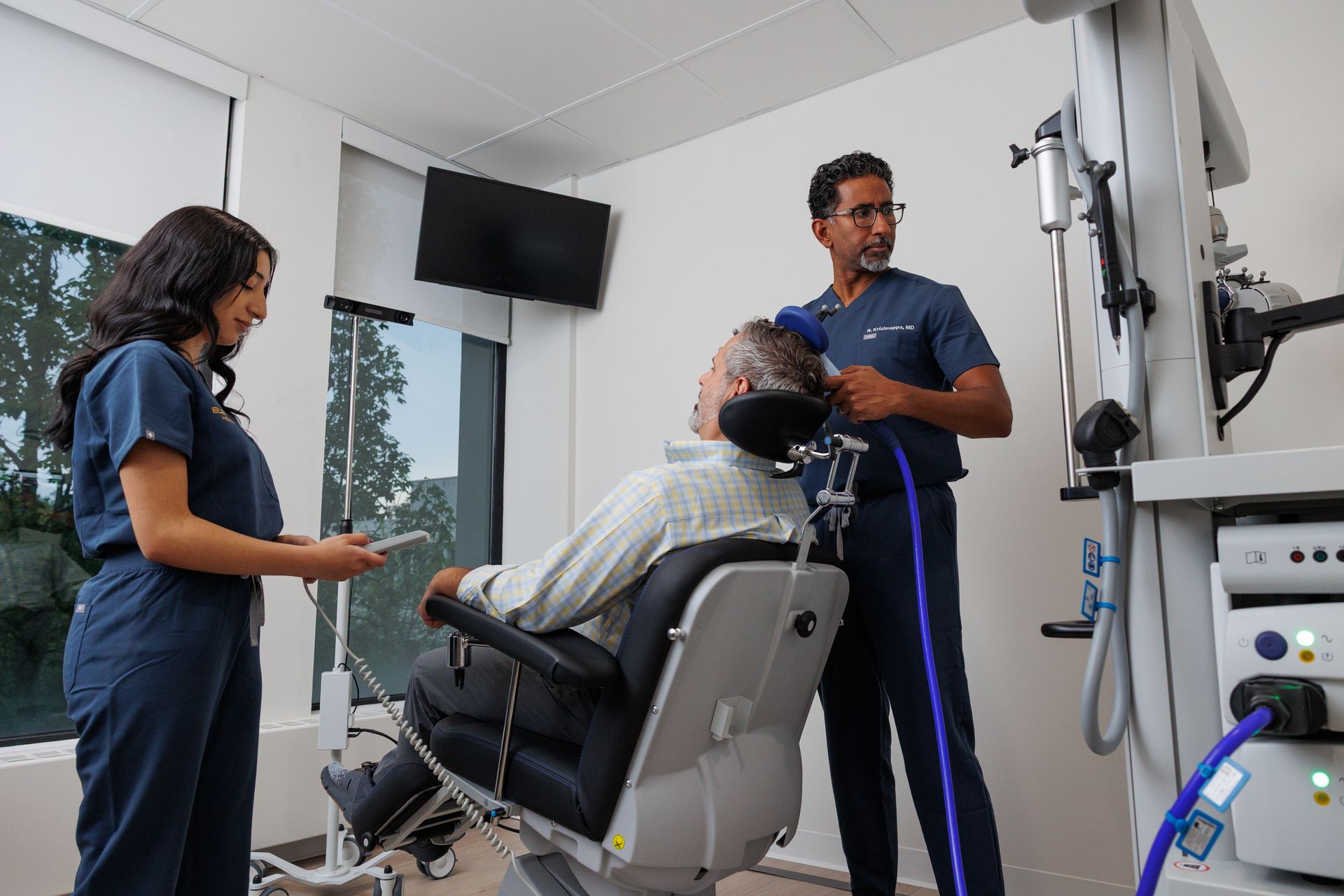
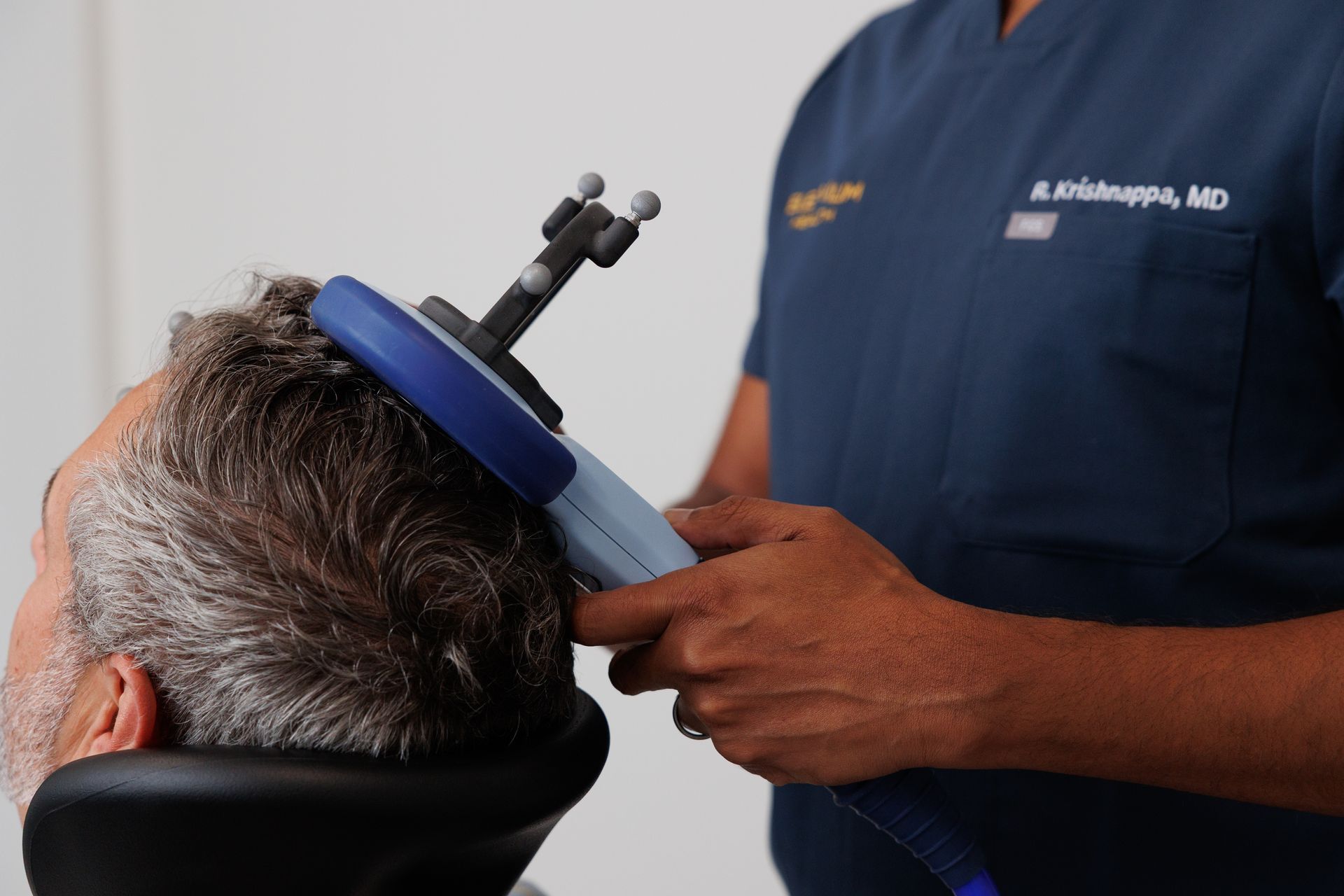
TMS (Transcranial Magnetic Stimulation)
TMS uses a magnetic coil placed on the scalp to stimulate brain circuits involved in mood regulation. It’s non-invasive, done awake, and doesn’t require anesthesia. In youth research, the most common side effects are typically headache and scalp discomfort, and serious adverse events are uncommon when safety guidelines are followed.
Spravato (Esketamine)
Spravato is a controlled substance nasal spray administered under supervision in a certified healthcare setting with monitoring because of risks like sedation/dissociation and respiratory depression. It is not dispensed for home use. Spravato. For pediatric use, labeling states that the safety/effectiveness has not been established.
Eligibility: Who might be a candidate?
TMS eligibility (typical teen considerations)
A teen may be considered when:
- Depression is moderate to severe and significantly impairing.
- Symptoms haven’t improved enough with evidence-based first-line care (therapy and/or medication), or there’s a reason meds aren’t a good fit.
- The teen can attend frequent sessions and cooperate with treatment.
No contraindications (for example, certain metal implants near the head).
Important age note: Some TMS devices now have FDA-cleared indications for adolescents 15–21 as adjunct treatment for MDD.
Spravato eligibility for teens
In practice, most reputable programs treat Spravato as an adult-focused option because pediatric safety/effectiveness is not established in the labeling. If a clinic considers ketamine/esketamine-type therapies for youth at all, it’s usually in highly specialized contexts with careful risk/benefit review and parent/guardian involvement.
Safety data: what parents should know
- Common: scalp discomfort, headache, fatigue - often improves after the first week.
- Rare but serious: seizure is the most discussed risk; it’s uncommon when patients are properly screened and protocols are followed (your clinician should review personal/family seizure history and medications that lower seizure threshold).
- Evidence base: Systematic reviews/meta-analyses in youth with depression generally report that rTMS appears reasonably safe and potentially effective, while also noting that more large, high-quality pediatric trials are still needed.
- Regulatory note: FDA clearance for adolescent MDD has been supported by real-world datasets for specific systems.
Spravato safety (and why monitoring matters)
- Requires clinic monitoring for at least 2 hours after each dose due to sedation/dissociation/respiratory depression concerns and other vital sign changes.
- Pediatric labeling: safety/effectiveness not established in pediatric patients.
Options Elevium may offer for younger patients
(Your exact offerings depend on Elevium’s clinical policies, clinician licensure, and local regulations—so frame this as “may include.”)
A) Comprehensive teen evaluation (the “starting point”)
- Psychiatric assessment, symptom scales, and review of prior treatments
- Screening for bipolar disorder, psychosis, substance use, ADHD/anxiety, trauma, and medical contributors
- Suicide risk assessment + safety planning
B) Evidence-based first-line care (often required before neuromodulation)
- Therapy options (CBT/DBT-informed approaches, family sessions)
- Medication management when appropriate
- Coordination with pediatrician and/or school supports
C) TMS (for eligible adolescents, commonly 15–21)
- If clinically appropriate and consistent with device indications and medical judgment
- Parent/guardian consent + teen assent
- Ongoing monitoring of mood, sleep, anxiety, and safety
Learn more about TMS for Teens
D) Spravato discussions (usually adult-focused)
- Many clinics will explain why Spravato is generally not a teen option because pediatric safety/effectiveness aren’t established
- If discussed at all, it should come with a very clear explanation of monitoring requirements and risk profile Spravato
E) Alternatives if a teen isn’t a candidate
- Medication optimization (including careful sequencing/augmentation)
- Higher level of care: intensive outpatient (IOP), partial hospitalization (PHP), or inpatient when needed
- For certain severe cases, referral pathways to academic centers or specialty programs
Will TMS change my teen’s personality?
TMS aims to reduce depressive symptoms by modulating brain circuits involved in mood. Parents typically notice changes like improved energy, reduced hopelessness, and better functioning - not a “new personality.” Monitoring is important to track mood shifts and agitation.
Does TMS hurt?
Many teens describe tapping/tingling on the scalp. Headache or scalp soreness can happen early on, and often improves.
How soon will we see improvement with TMS?
Some notice changes within 2–3 weeks; others need a full course. Your clinic should set expectations and measure progress with symptom scales.
Is Spravato safe for teens?
Spravato’s safety/effectiveness is not established in pediatric patients, so most programs do not use it routinely under 18.
If my teen has suicidal thoughts, is TMS/Spravato the right first step?
Suicidal thoughts require immediate clinical assessment. Sometimes the right first step is a safety plan, urgent psychiatry, crisis services, and possibly a higher level of care while longer-term treatments are planned.
Share Article